1. Recombinant Protein/Monoclonal Antibody Production
Cell Culture Medium Preparation
- Dissolve culture medium powders (amino acids, vitamins, salts) in WFI filtered through a 0.22μm terminal filter to achieve an osmolarity range of 280–320 mOsmol/kg, preventing cell death from osmotic stress.
- Use WFI with TOC <5 ppb to prepare buffers like PBS, avoiding organic contaminants that could interfere with protein expression.
Equipment Configuration: Dual-stage reverse osmosis + electrodeionization (EDI) polishing + UV sterilization + 0.22μm cartridge filters, delivering water resistivity ≥18.2 MΩ·cm.
Chromatography Processes
- During affinity/ion-exchange chromatography column regeneration, perform gradient salt concentration exchange using WFI to prevent impurity carryover into resins.
- For viral clearance via low-temperature pasteurization, preheat WFI to 60°C before treating harvested material, while monitoring conductivity to detect potential media leakage.
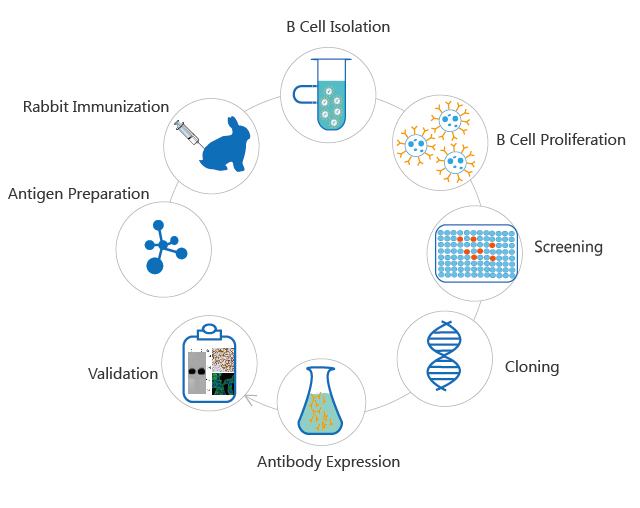
2. Vaccine Manufacturing
Viral Culture Media Preparation
- Dilute allantoic fluid from chicken embryos with WFI to adjust influenza virus titer, maintaining aseptic conditions in bioreactors via online ozone sanitization.
- Supply thermostatic WFI (37±0.5°C) continuously during perfusion culture of Vero cells for rabies vaccine production.
Adjuvant Formulation
- Use WFI as the dispersion phase when preparing aluminum adjuvant suspensions, combined with high-pressure microfluidization to achieve nanoparticle homogeneity.
- Deoxygenate WFI via nitrogen bubbling before lipid nanoparticle (LNP) synthesis for mRNA vaccines, preventing phospholipid oxidation.

3. Cell Therapy Products (CAR-T/Stem Cells)
Cryoprotectant Solution Preparation
- Dilute dimethyl sulfoxide (DMSO) to 10% final concentration using WFI for cryopreservation solutions, avoiding sodium ion interference with cell viability.
- Wash centrifuge tubes thrice with WFI during T-cell activation medium changes to remove serum residues.
Closed-System Operations
- Flush tubing inner surfaces with WFI during aseptic docking of bags to centrifuges, eliminating open manipulation risks.
- Resuspend recombinant insulin in WFI for Xeno-free stem cell culture media formulation.
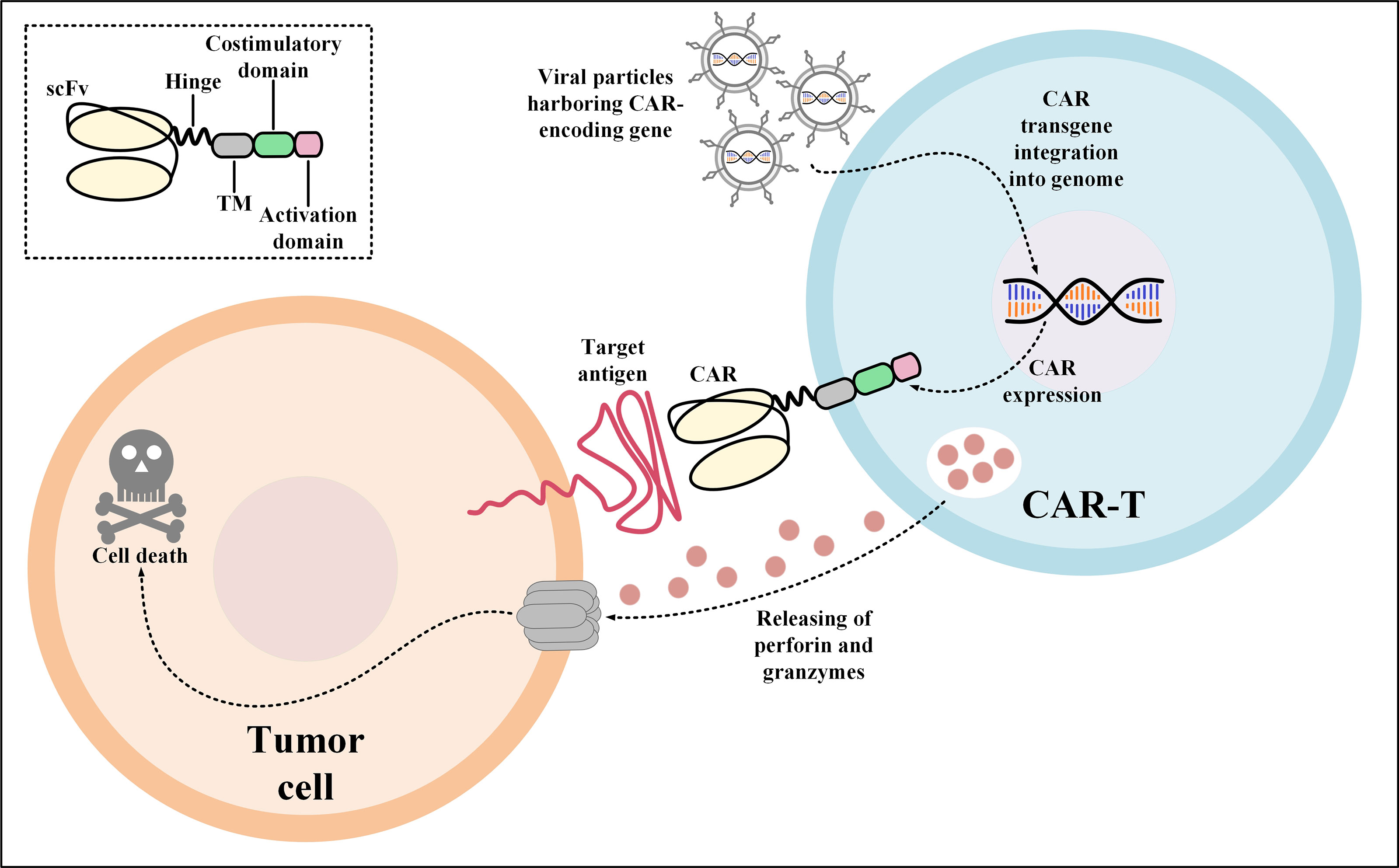
4. Plasma Derivatives Production
Plasma Fractionation
- Adjust ethanol concentration in cold ethanol precipitation for albumin extraction using WFI, ensuring precise critical precipitation points.
- Formulate dialysis buffers with WFI-added sodium chloride for factor VIII purification, controlling ionic strength to prevent denaturation.
Virus Removal Validation
- Pre-filter raw material through 0.1μm filters before nanofiltration to prevent membrane fouling.
- Maintain pH 3.5–3.8 during low-pH incubation for enveloped virus inactivation using WFI-adjusted buffer systems.

5. Gene Therapy Drug Production
AAV Vector Purification
- Create cesium chloride density gradients using WFI for AAV purification via ultracentrifugation.
- Diafiltrate concentrated viral vectors against WFI to reduce host cell protein residues.
Plasmid DNA Fermentation
- Feed E. coli high-density cultures with WFI-prepared glucose/magnesium solutions by bioreactor to avoid metabolic byproduct accumulation.
- Backflush depth filters with WFI post-cell lysate clarification to improve yield.
6. Ophthalmic/Topical Formulations
Eye Drop Filling
- Cool WFI to 2–8°C under nitrogen blanket for hyaluronic acid eye drop production, preventing polysaccharide degradation.
- Direct-connect stainless steel corrugated hoses from WFI storage to filling needles under ISO Class 5 laminar flow.
Gel Base Preparation
- Disperse carbomer polymers in WFI for ophthalmic gel bases, avoiding calcium/magnesium ion-induced crosslinking failure.
- Osmotically balance nasal sprays using WFI-adjusted sodium chloride concentrations to 290 mOsmol/kg.
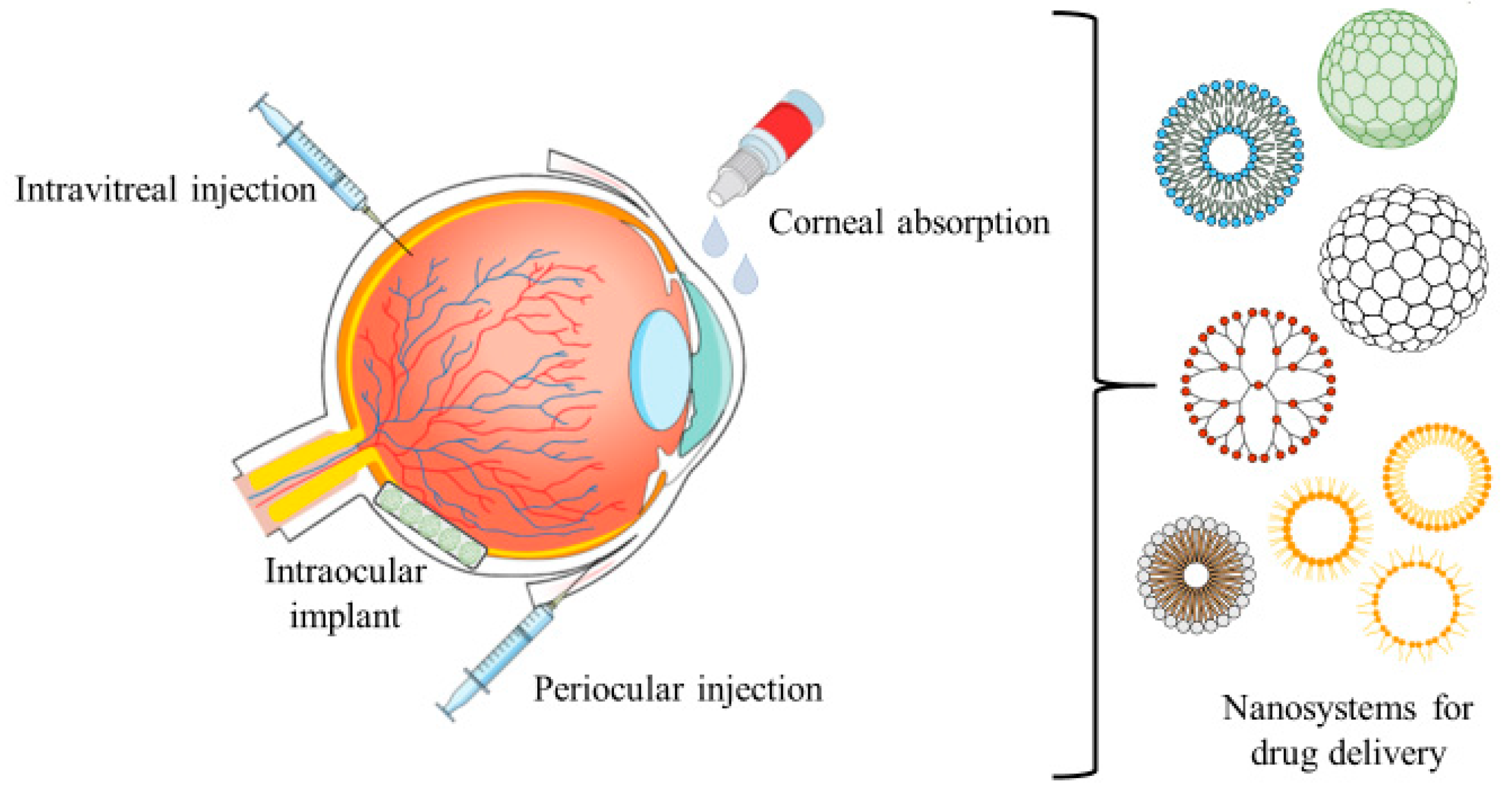
7. Specialized Drug Delivery Systems
Lipid Nanoparticle Carriers
- Form LNPs via ethanol injection method using WFI for mRNA vaccine encapsulation, targeting Z-average particle sizes <100 nm.
- Prepare trehalose cryoprotectants in WFI for lipid nanoparticle stabilization.
Implantable Microspheres
- Wash poly(lactic-co-glycolic acid) (PLGA) microspheres with WFI to remove polyvinylpyrrolidone (PVP) stabilizers prior to drug loading.
- Conduct extractables studies on sterilized radioactive embolic microspheres stored in WFI.

These application examples demonstrate how WFI systems are precisely engineered to meet diverse biopharmaceutical process requirements, directly impacting product efficacy and safety through controlled solvent supply and critical parameter management.







